Research and Development
iCoat Medical’s products are based on 25 years’ research and development in close collaboration with leading academic institutions. Pre-clinical studies unanimously show that iCM012 improves the kidney’s function in connection to transplantation. Initially iCM012 will be evaluated for kidneys but the ambition is that the product can be used in connection to all other types of transplantation.

Ischemia Reperfusion Injury
Ischemia Reperfusion Injury (IRI) is a mechanism that leads to tromboinflammation and tissue injury in connection to several clinical conditions and diseases. IRI can arise in connection to: organ and cell transplantion, thromboembolic disease such as cardiac infarction (heart attack), and stroke and open heart surgery using cardiopulmonary bypass (heart-lung machine).
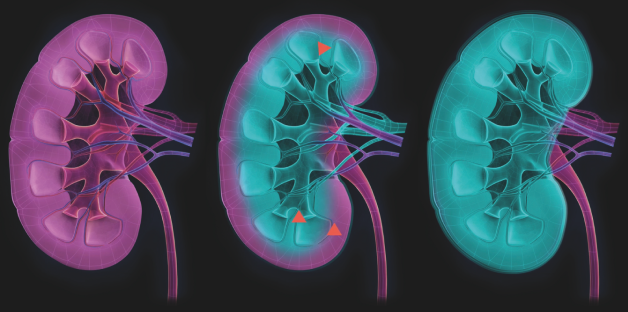
Coating technology
iCoat Medical’s products utilize a proprietary coating technology. The coating technology enables various molecules to spontaneously enter into cell membranes and form a tight coat that prevents plasma proteins and cells from reacting with the cell surface. iCoat Medical applies this technology to specifically protect ischemic cells from attack from the innate immune system.
iCM012 & iCM020
iCM012 is a modified polymer that has the ability to enter a cell membrane and form a protective barrier which prevents plasma proteins and immune cells from reacting with the cell membrane. iCM020 is such a molecule that is developed to prevent damage particularly induced by warm ischemia by creating a protective barrier on the cell membrane.
Orphan Drugs
In order to support development of treatments for patients that suffer from rare diseases and where there is an unmet medical need, authorities all over the world have introduced the classification Orphan Drugs.